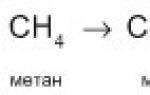
X-ray length. Basic properties of X-ray radiation
Modern medical diagnosis and treatment of certain diseases cannot be imagined without devices that use the properties of x-ray radiation. The discovery of X-rays occurred more than 100 years ago, but even now work continues on the creation of new techniques and devices to minimize the negative effects of radiation on the human body.
Who discovered X-rays and how?
Under natural conditions, X-ray fluxes are rare and are emitted only by certain radioactive isotopes. X-rays or X-rays were only discovered in 1895 by the German scientist Wilhelm Röntgen. This discovery occurred by chance, during an experiment to study the behavior of light rays in conditions approaching a vacuum. The experiment involved a cathode gas-discharge tube with reduced pressure and a fluorescent screen, which each time began to glow the moment the tube began to operate.
Interested in the strange effect, Roentgen conducted a series of studies showing that the resulting radiation, invisible to the eye, is capable of penetrating through various obstacles: paper, wood, glass, some metals, and even through the human body. Despite the lack of understanding of the very nature of what is happening, whether such a phenomenon is caused by the generation of a stream of unknown particles or waves, the following pattern was noted - radiation easily passes through the soft tissues of the body, and much harder through hard living tissues and non-living substances.
Roentgen was not the first to study this phenomenon. In the mid-19th century, similar possibilities were explored by the Frenchman Antoine Mason and the Englishman William Crookes. However, it was Roentgen who first invented a cathode tube and an indicator that could be used in medicine. He was the first to publish a scientific work, which earned him the title of the first Nobel laureate among physicists.
In 1901, a fruitful collaboration between three scientists began, who became the founding fathers of radiology and radiology.

Properties of X-rays
X-rays are a component of the general spectrum of electromagnetic radiation. The wavelength lies between gamma and ultraviolet rays. X-rays have all the usual wave properties:
- diffraction;
- refraction;
- interference;
- speed of propagation (it is equal to light).
To artificially generate a flux of X-rays, special devices are used - X-ray tubes. X-ray radiation occurs due to the contact of fast electrons from tungsten with substances evaporating from the hot anode. Against the background of interaction, electromagnetic waves of short length appear, located in the spectrum from 100 to 0.01 nm and in the energy range of 100-0.1 MeV. If the wavelength of the rays is less than 0.2 nm, this is hard radiation; if the wavelength is greater than this value, they are called soft X-rays.
It is significant that the kinetic energy arising from the contact of electrons and the anode substance is 99% converted into heat energy and only 1% is X-rays.
X-ray radiation – bremsstrahlung and characteristic
X-radiation is a superposition of two types of rays - bremsstrahlung and characteristic. They are generated in the tube simultaneously. Therefore, X-ray irradiation and the characteristics of each specific X-ray tube - its radiation spectrum - depend on these indicators and represent their overlap.
Bremsstrahlung or continuous X-rays are the result of the deceleration of electrons evaporated from a tungsten filament.
Characteristic or line X-ray rays are formed at the moment of restructuring of the atoms of the substance of the anode of the X-ray tube. The wavelength of the characteristic rays directly depends on the atomic number of the chemical element used to make the anode of the tube.
The listed properties of X-rays allow them to be used in practice:
- invisibility to ordinary eyes;
- high penetrating ability through living tissues and non-living materials that do not transmit rays of the visible spectrum;
- ionization effect on molecular structures.
Principles of X-ray imaging
The properties of X-rays on which imaging is based is the ability to either decompose or cause the glow of certain substances.
X-ray irradiation causes a fluorescent glow in cadmium and zinc sulfides - green, and in calcium tungstate - blue. This property is used in medical x-ray imaging techniques and also increases the functionality of x-ray screens.
The photochemical effect of X-rays on photosensitive silver halide materials (exposure) allows for diagnostics - taking X-ray photographs. This property is also used when measuring the total dose received by laboratory assistants in X-ray rooms. Body dosimeters contain special sensitive tapes and indicators. The ionizing effect of X-ray radiation makes it possible to determine the qualitative characteristics of the resulting X-rays.
A single exposure to radiation from conventional X-rays increases the risk of cancer by only 0.001%.
Areas where X-rays are used
The use of X-rays is permissible in the following industries:
- Safety. Stationary and portable devices for detecting dangerous and prohibited items at airports, customs or in crowded places.
- Chemical industry, metallurgy, archeology, architecture, construction, restoration work - to detect defects and conduct chemical analysis of substances.
- Astronomy. Helps to observe cosmic bodies and phenomena using X-ray telescopes.
- Military industry. To develop laser weapons.
The main application of X-ray radiation is in the medical field. Today, the section of medical radiology includes: radiodiagnosis, radiotherapy (x-ray therapy), radiosurgery. Medical universities graduate highly specialized specialists – radiologists.
X-Radiation - harm and benefits, effects on the body
The high penetrating power and ionizing effect of X-rays can cause changes in the structure of cell DNA, and therefore pose a danger to humans. The harm from x-rays is directly proportional to the radiation dose received. Different organs respond to radiation to varying degrees. The most susceptible include:
- bone marrow and bone tissue;
- lens of the eye;
- thyroid;
- mammary and reproductive glands;
- lung tissue.
Uncontrolled use of X-ray irradiation can cause reversible and irreversible pathologies.
Consequences of X-ray irradiation:
- damage to the bone marrow and the occurrence of pathologies of the hematopoietic system - erythrocytopenia, thrombocytopenia, leukemia;
- damage to the lens, with subsequent development of cataracts;
- cellular mutations that are inherited;
- development of cancer;
- receiving radiation burns;
- development of radiation sickness.
Important! Unlike radioactive substances, X-rays do not accumulate in body tissues, which means that X-rays do not need to be removed from the body. The harmful effect of X-ray radiation ends when the medical device is turned off.
The use of X-ray radiation in medicine is permissible not only for diagnostic (traumatology, dentistry), but also for therapeutic purposes:
- X-rays in small doses stimulate metabolism in living cells and tissues;
- certain limiting doses are used for the treatment of oncological and benign neoplasms.

Methods for diagnosing pathologies using X-rays
Radiodiagnostics includes the following techniques:
- Fluoroscopy is a study during which an image is obtained on a fluorescent screen in real time. Along with the classic acquisition of an image of a body part in real time, today there are X-ray television transillumination technologies - the image is transferred from a fluorescent screen to a television monitor located in another room. Several digital methods have been developed for processing the resulting image, followed by transferring it from the screen to paper.
- Fluorography is the cheapest method of examining the chest organs, which consists of taking a reduced-scale image of 7x7 cm. Despite the likelihood of error, it is the only way to conduct a mass annual examination of the population. The method is not dangerous and does not require removal of the received radiation dose from the body.
- Radiography is the production of a summary image on film or paper to clarify the shape of an organ, its position or tone. Can be used to assess peristalsis and the condition of mucous membranes. If there is a choice, then among modern X-ray devices, preference should be given neither to digital devices, where the x-ray flux can be higher than that of old devices, but to low-dose X-ray devices with direct flat semiconductor detectors. They allow you to reduce the load on the body by 4 times.
- Computed X-ray tomography is a technique that uses X-rays to obtain the required number of images of sections of a selected organ. Among the many varieties of modern CT devices, low-dose high-resolution computed tomographs are used for a series of repeated studies.

Radiotherapy
X-ray therapy is a local treatment method. Most often, the method is used to destroy cancer cells. Since the effect is comparable to surgical removal, this treatment method is often called radiosurgery.
Today, x-ray treatment is carried out in the following ways:
- External (proton therapy) – a radiation beam enters the patient’s body from the outside.
- Internal (brachytherapy) - the use of radioactive capsules by implanting them into the body, placing them closer to the cancer tumor. The disadvantage of this method of treatment is that until the capsule is removed from the body, the patient needs to be isolated.
These methods are gentle, and their use is preferable to chemotherapy in some cases. This popularity is due to the fact that the rays do not accumulate and do not require removal from the body; they have a selective effect, without affecting other cells and tissues.
Safe exposure limit to X-rays
This indicator of the norm of permissible annual exposure has its own name - genetically significant equivalent dose (GSD). This indicator does not have clear quantitative values.
- This indicator depends on the patient’s age and desire to have children in the future.
- Depends on which organs were examined or treated.
- The GZD is influenced by the level of natural radioactive background in the region where a person lives.
Today the following average GZD standards are in effect:
- the level of exposure from all sources, with the exception of medical ones, and without taking into account the natural background radiation - 167 mrem per year;
- the norm for an annual medical examination is not higher than 100 mrem per year;
- the total safe value is 392 mrem per year.
X-ray radiation does not require removal from the body, and is dangerous only in case of intense and prolonged exposure. Modern medical equipment uses low-energy irradiation of short duration, so its use is considered relatively harmless.
X-RAY
X-ray radiation occupies the region of the electromagnetic spectrum between gamma and ultraviolet radiation and is electromagnetic radiation with a wavelength from 10 -14 to 10 -7 m. In medicine, X-ray radiation with a wavelength from 5 x 10 -12 to 2.5 x 10 -10 is used m, that is, 0.05 - 2.5 angstroms, and for X-ray diagnostics itself - 0.1 angstroms. Radiation is a stream of quanta (photons) propagating linearly at the speed of light (300,000 km/s). These quanta have no electrical charge. The mass of a quantum is an insignificant part of an atomic mass unit.
Energy of quanta measured in Joules (J), but in practice they often use a non-systemic unit "electron-volt" (eV) . One electron volt is the energy that one electron acquires when passing through a potential difference of 1 volt in an electric field. 1 eV = 1.6 10~ 19 J. The derivatives are the kiloelectron-volt (keV), equal to a thousand eV, and the megaelectron-volt (MeV), equal to a million eV.
X-rays are produced using X-ray tubes, linear accelerators and betatrons. In an X-ray tube, the potential difference between the cathode and the target anode (tens of kilovolts) accelerates the electrons bombarding the anode. X-ray radiation occurs when fast electrons are decelerated in the electric field of the atoms of the anode substance (bremsstrahlung) or during the restructuring of the inner shells of atoms (characteristic radiation) . Characteristic X-ray radiation has a discrete nature and occurs when the electrons of the atoms of the anode substance transfer from one energy level to another under the influence of external electrons or radiation quanta. Bremsstrahlung X-rays has a continuous spectrum depending on the anode voltage on the X-ray tube. When braking in the anode substance, electrons spend most of their energy on heating the anode (99%) and only a small fraction (1%) is converted into X-ray energy. In X-ray diagnostics, bremsstrahlung radiation is most often used.
The basic properties of X-rays are characteristic of all electromagnetic radiation, but there are some special features. X-rays have the following properties:
- invisibility - sensitive cells of the human retina do not respond to X-rays, since their wavelength is thousands of times shorter than that of visible light;
- straight propagation – rays are refracted, polarized (propagated in a certain plane) and diffracted, like visible light. The refractive index differs very little from unity;
- penetrating power - penetrate without significant absorption through significant layers of substances opaque to visible light. The shorter the wavelength, the greater the penetrating power of x-rays;
- absorption capacity - have the ability to be absorbed by body tissues; all x-ray diagnostics are based on this. The absorption capacity depends on the specific gravity of the tissue (the higher, the greater the absorption); on the thickness of the object; on the radiation hardness;
- photographic action - decompose silver halide compounds, including those found in photographic emulsions, which makes it possible to obtain X-ray images;
- luminescent effect - cause luminescence of a number of chemical compounds (luminophores), the X-ray transillumination technique is based on this. The intensity of the glow depends on the structure of the fluorescent substance, its quantity and distance from the X-ray source. Phosphors are used not only to obtain images of objects under study on a fluoroscopic screen, but also in radiography, where they make it possible to increase the radiation exposure to the radiographic film in the cassette due to the use of intensifying screens, the surface layer of which is made of fluorescent substances;
- ionization effect - have the ability to cause the disintegration of neutral atoms into positively and negatively charged particles, dosimetry is based on this. The effect of ionization of any medium is the formation in it of positive and negative ions, as well as free electrons from neutral atoms and molecules of the substance. Ionization of the air in the X-ray room during operation of the X-ray tube leads to an increase in the electrical conductivity of the air and an increase in static electric charges on cabinet objects. In order to eliminate such undesirable effects, forced supply and exhaust ventilation is provided in X-ray rooms;
- biological effect - have an impact on biological objects, in most cases this impact is harmful;
- inverse square law - for a point source of X-ray radiation, the intensity decreases in proportion to the square of the distance to the source.
In 1895, the German physicist Roentgen, conducting experiments on the passage of current between two electrodes in a vacuum, discovered that a screen covered with a luminescent substance (barium salt) glows, although the discharge tube is covered with a black cardboard screen - this is how radiation penetrating through opaque barriers, called X-rays X-rays. It was discovered that X-ray radiation, invisible to humans, is absorbed in opaque objects the more strongly, the higher the atomic number (density) of the barrier, so X-rays easily pass through the soft tissues of the human body, but are retained by the bones of the skeleton. Sources of powerful X-rays have been designed to make it possible to illuminate metal parts and find internal defects in them.
The German physicist Laue suggested that X-rays are the same electromagnetic radiation as visible light rays, but with a shorter wavelength and all the laws of optics apply to them, including the possibility of diffraction. In visible light optics, diffraction at an elementary level can be represented as the reflection of light from a system of lines - a diffraction grating, which occurs only at certain angles, and the angle of reflection of the rays is related to the angle of incidence, the distance between the lines of the diffraction grating and the wavelength of the incident radiation. For diffraction to occur, the distance between the lines must be approximately equal to the wavelength of the incident light.
Laue suggested that X-rays have a wavelength close to the distance between individual atoms in crystals, i.e. the atoms in the crystal create a diffraction grating for x-rays. X-rays directed at the surface of the crystal were reflected onto the photographic plate, as predicted by theory.
Any changes in the position of atoms affect the diffraction pattern, and by studying X-ray diffraction, one can find out the arrangement of atoms in a crystal and the change in this arrangement under any physical, chemical and mechanical influences on the crystal.
Nowadays, X-ray analysis is used in many fields of science and technology; with its help, the arrangement of atoms in existing materials has been determined and new materials have been created with a given structure and properties. Recent advances in this field (nanomaterials, amorphous metals, composite materials) create a field of activity for the next scientific generations.
Occurrence and properties of X-ray radiation
The source of X-rays is an X-ray tube, which has two electrodes - a cathode and an anode. When the cathode is heated, electron emission occurs; electrons escaping from the cathode are accelerated by the electric field and strike the surface of the anode. What distinguishes an X-ray tube from a conventional radio tube (diode) is mainly its higher accelerating voltage (more than 1 kV).
When an electron leaves the cathode, the electric field forces it to fly towards the anode, while its speed continuously increases; the electron carries a magnetic field, the strength of which increases with increasing speed of the electron. Reaching the anode surface, the electron is sharply decelerated, and an electromagnetic pulse with wavelengths in a certain interval appears (bremsstrahlung). The distribution of radiation intensity over wavelengths depends on the anode material of the X-ray tube and the applied voltage, while on the short wave side this curve begins with a certain threshold minimum wavelength, depending on the applied voltage. The combination of rays with all possible wavelengths forms a continuous spectrum, and the wavelength corresponding to the maximum intensity is 1.5 times the minimum wavelength.
As the voltage increases, the X-ray spectrum changes dramatically due to the interaction of atoms with high-energy electrons and quanta of primary X-rays. An atom contains internal electron shells (energy levels), the number of which depends on the atomic number (denoted by the letters K, L, M, etc.) Electrons and primary X-rays knock electrons out of one energy level to another. A metastable state arises and for the transition to a stable state a jump of electrons in the opposite direction is necessary. This jump is accompanied by the release of an energy quantum and the appearance of X-ray radiation. Unlike X-rays with a continuous spectrum, this radiation has a very narrow range of wavelengths and high intensity (characteristic radiation) ( cm. rice.). The number of atoms that determine the intensity of the characteristic radiation is very large; for example, for an X-ray tube with a copper anode at a voltage of 1 kV and a current of 15 mA, 10 14 –10 15 atoms produce characteristic radiation in 1 s. This value is calculated as the ratio of the total power of X-ray radiation to the energy of an X-ray quantum from the K-shell (K-series of X-ray characteristic radiation). The total power of X-ray radiation is only 0.1% of the power consumption, the rest is lost mainly due to conversion to heat.
Due to their high intensity and narrow wavelength range, characteristic X-rays are the main type of radiation used in scientific research and process control. Simultaneously with the K-series rays, L and M-series rays are generated, which have significantly longer wavelengths, but their use is limited. The K-series has two components with close wavelengths a and b, while the intensity of the b-component is 5 times less than a. In turn, the a-component is characterized by two very close wavelengths, the intensity of one of which is 2 times greater than the other. To obtain radiation with one wavelength (monochromatic radiation), special methods have been developed that use the dependence of absorption and diffraction of x-rays on wavelength. An increase in the atomic number of an element is associated with a change in the characteristics of the electron shells, and the higher the atomic number of the X-ray tube anode material, the shorter the K-series wavelength. The most widely used are tubes with anodes made of elements with atomic numbers from 24 to 42 (Cr, Fe, Co, Cu, Mo) and wavelengths from 2.29 to 0.712 A (0.229 - 0.712 nm).
In addition to the X-ray tube, sources of X-ray radiation can be radioactive isotopes, some can directly emit X-rays, others emit electrons and a-particles that generate X-rays when bombarding metal targets. The intensity of X-ray radiation from radioactive sources is usually much less than an X-ray tube (with the exception of radioactive cobalt, which is used in flaw detection and produces radiation of a very short wavelength - g-radiation), they are small in size and do not require electricity. Synchrotron X-rays are produced in electron accelerators; the wavelength of this radiation is significantly longer than that obtained in X-ray tubes (soft X-rays), and its intensity is several orders of magnitude higher than the radiation intensity of X-ray tubes. There are also natural sources of X-ray radiation. Radioactive impurities have been found in many minerals, and X-ray emission from space objects, including stars, has been recorded.
Interaction of X-rays with crystals
In X-ray studies of materials with a crystalline structure, interference patterns resulting from the scattering of X-rays by electrons belonging to the atoms of the crystal lattice are analyzed. Atoms are considered immobile, their thermal vibrations are not taken into account, and all electrons of the same atom are considered concentrated at one point - a node of the crystal lattice.
To derive the basic equations for X-ray diffraction in a crystal, the interference of rays scattered by atoms located along a straight line in the crystal lattice is considered. A plane wave of monochromatic X-ray radiation falls on these atoms at an angle whose cosine is equal to a 0 . The laws of interference of rays scattered by atoms are similar to those existing for a diffraction grating, which scatters light radiation in the visible wavelength range. In order for the amplitudes of all vibrations to add up at a large distance from the atomic row, it is necessary and sufficient that the difference in the paths of the rays coming from each pair of neighboring atoms contains an integer number of wavelengths. When the distance between atoms A this condition looks like:
A(a – a 0) = h l,
where a is the cosine of the angle between the atomic row and the deflected beam, h – integer. In all directions that do not satisfy this equation, the rays do not propagate. Thus, scattered rays form a system of coaxial cones, the common axis of which is the atomic row. Traces of cones on a plane parallel to the atomic row are hyperbolas, and on a plane perpendicular to the row they are circles.
When rays are incident at a constant angle, polychromatic (white) radiation is decomposed into a spectrum of rays deflected at fixed angles. Thus, the atomic series is a spectrograph for x-rays.
Generalization to a two-dimensional (flat) atomic lattice, and then to a three-dimensional volumetric (spatial) crystal lattice gives two more similar equations, which include the angles of incidence and reflection of X-ray radiation and the distances between atoms in three directions. These equations are called Laue's equations and form the basis of X-ray diffraction analysis.
The amplitudes of rays reflected from parallel atomic planes add up, etc. the number of atoms is very large, the reflected radiation can be detected experimentally. The reflection condition is described by the Wulff–Bragg equation2d sinq = nl, where d is the distance between adjacent atomic planes, q is the grazing angle between the direction of the incident beam and these planes in the crystal, l is the wavelength of the x-ray radiation, n is an integer called the order of reflection. Angle q is the angle of incidence with respect specifically to atomic planes, which do not necessarily coincide in direction with the surface of the sample under study.
Several methods of X-ray diffraction analysis have been developed, using both radiation with a continuous spectrum and monochromatic radiation. The object under study can be stationary or rotating, can consist of one crystal (single crystal) or many (polycrystal); diffracted radiation can be recorded using a flat or cylindrical X-ray film or an X-ray detector moving around the circumference, but in all cases during the experiment and interpretation of the results, the Wulff–Bragg equation is used.
X-ray analysis in science and technology
With the discovery of X-ray diffraction, researchers had at their disposal a method that made it possible, without a microscope, to study the arrangement of individual atoms and changes in this arrangement under external influences.
The main application of X-rays in fundamental science is structural analysis, i.e. establishing the spatial arrangement of individual atoms in a crystal. To do this, single crystals are grown and X-ray analysis is performed, studying both the locations and intensities of the reflections. The structures of not only metals, but also complex organic substances, in which the unit cells contain thousands of atoms, have now been determined.
In mineralogy, the structures of thousands of minerals have been determined using X-ray analysis and express methods for analyzing mineral raw materials have been created.
Metals have a relatively simple crystal structure and the X-ray method makes it possible to study its changes during various technological treatments and create the physical basis of new technologies.
The phase composition of the alloys is determined by the location of the lines on the X-ray diffraction patterns, the number, size and shape of crystals are determined by their width, and the orientation of the crystals (texture) is determined by the intensity distribution in the diffraction cone.
Using these techniques, processes during plastic deformation are studied, including crystal fragmentation, the occurrence of internal stresses and imperfections in the crystal structure (dislocations). When deformed materials are heated, stress relief and crystal growth (recrystallization) are studied.
X-ray analysis of alloys determines the composition and concentration of solid solutions. When a solid solution appears, the interatomic distances and, consequently, the distances between atomic planes change. These changes are small, so special precision methods have been developed for measuring the periods of the crystal lattice with an accuracy two orders of magnitude greater than the measurement accuracy using conventional x-ray research methods. The combination of precision measurements of crystal lattice periods and phase analysis makes it possible to construct the boundaries of phase regions in the phase diagram. The X-ray method can also detect intermediate states between solid solutions and chemical compounds - ordered solid solutions in which the impurity atoms are not randomly located, as in solid solutions, and at the same time not with three-dimensional order, as in chemical compounds. X-ray diffraction patterns of ordered solid solutions contain additional lines; interpretation of the x-ray diffraction patterns shows that impurity atoms occupy certain places in the crystal lattice, for example, at the vertices of a cube.
When an alloy that does not undergo phase transformations is quenched, a supersaturated solid solution may arise, and upon further heating or even holding at room temperature, the solid solution decomposes with the release of particles of a chemical compound. This is the effect of aging and it appears on x-rays as a change in the position and width of the lines. Aging research is especially important for non-ferrous metal alloys, for example, aging transforms a soft, hardened aluminum alloy into the durable structural material duralumin.
X-ray studies of steel heat treatment are of greatest technological importance. When quenching (rapid cooling) of steel, a diffusion-free austenite-martensite phase transition occurs, which leads to a change in structure from cubic to tetragonal, i.e. the unit cell takes the shape of a rectangular prism. On radiographs this appears as widening of the lines and the division of some lines into two. The reasons for this effect are not only a change in the crystal structure, but also the occurrence of large internal stresses due to the thermodynamic nonequilibrium of the martensitic structure and sudden cooling. When tempering (heating the hardened steel), the lines on the x-ray diffraction patterns narrow, this is associated with a return to the equilibrium structure.
In recent years, X-ray studies of the processing of materials with concentrated energy flows (laser beams, shock waves, neutrons, electron pulses) have acquired great importance; they required new techniques and produced new X-ray effects. For example, when laser beams act on metals, heating and cooling occur so quickly that during cooling, crystals in the metal only have time to grow to sizes of several elementary cells (nanocrystals) or do not have time to arise at all. After cooling, such a metal looks like ordinary metal, but does not give clear lines on the X-ray diffraction pattern, and the reflected X-rays are distributed over the entire range of grazing angles.
After neutron irradiation, additional spots (diffuse maxima) appear on x-ray diffraction patterns. Radioactive decay also causes specific X-ray effects associated with changes in structure, as well as the fact that the sample under study itself becomes a source of X-ray radiation.
Just like most of the great discoveries in human history, X-rays were discovered by accident. In 1895, German scientist Wilhelm Roentgen made a discovery while conducting an experiment with electron beams in a gas discharge tube. Roentgen noticed that the fluorescent screen in his laboratory began to glow while the electron flow was turned on. This would be quite a common occurrence, since fluorescent material should glow under the influence of electromagnetic radiation, if not for one thing: the tube was fenced off from the screen with a dense black screen. Wilhelm suggested that this was caused by radiation.
Roentgen continued to experiment and placed various objects between the screen and the tube and the screen continued to glow. Finally, he stuck his hand in front of the tube and saw the silhouette of bones on the screen. Interest in his invention was immediately shown. This discovery is one of the most significant advances in medicine, as it allowed doctors to look inside a patient without performing surgery or even touching him.
X-rays have many similarities to ordinary visible light. Both are a stream of electromagnetic wave-like energy carried by particles called photons. The difference is the wavelength.
Visible light photons and X-ray photons are both the product of the movement of electrons in atoms. Electrons occupy different energy levels (orbitals) around the nucleus of an atom. When an electron moves from a high orbital to a lower one, some energy is released in the form of photons. The amount of energy of the released photons depends on how far the electron has moved, that is, how deep it has fallen. If a photon collides with another atom, the atom can absorb the photon's energy and move its electron(s) to a higher level if there is enough energy to do so.

The atoms that make up the tissues of the human body absorb photons of visible light very well. Their energy level is enough to transfer electrons to a higher level. Radio waves do not have enough energy to move electrons between orbits. At the same time, X-ray waves pass through things for a different reason: they have too much energy. Although they may lose some energy in order not just to transfer, but even to tear off electrons from atoms, most of the rays still pass through materials.
Heavy atoms, such as lead, are more likely to absorb X-rays because they require a lot of energy to transfer their electrons to outer levels. And light atoms, of which the tissues of our body are predominantly composed, have less chance of absorbing photons, since they have a much smaller distance between levels and they simply cannot accept (“overpower”) the high energy of X-rays. Calcium atoms are much larger than the chemical elements that make up other tissues, so they absorb some of the energy and appear lighter in photographs.
 As mentioned above, the most significant application of X-rays was found in the X-ray machine, the design of which strongly resembles the experiment carried out by their discoverer. At the heart of any X-ray machine is a source of X-rays. It, in turn, is a gas-filled tube with positive (cathode) and negative (anode) electrodes. The cathode is a filament, and the anode is a tungsten disk. When an electric current is passed through the filament, it heats up, releasing electrons from its surface. The anode, in turn, attracts them through the gaseous medium, resulting in a very large potential difference. The electrons that break through this large barrier, hitting the anode, knock out tungsten electrons from the upper energy levels directly to the lower ones, as a result of which a large portion of the energy is released in the form of a photon, which is a component of the X-ray flux.
As mentioned above, the most significant application of X-rays was found in the X-ray machine, the design of which strongly resembles the experiment carried out by their discoverer. At the heart of any X-ray machine is a source of X-rays. It, in turn, is a gas-filled tube with positive (cathode) and negative (anode) electrodes. The cathode is a filament, and the anode is a tungsten disk. When an electric current is passed through the filament, it heats up, releasing electrons from its surface. The anode, in turn, attracts them through the gaseous medium, resulting in a very large potential difference. The electrons that break through this large barrier, hitting the anode, knock out tungsten electrons from the upper energy levels directly to the lower ones, as a result of which a large portion of the energy is released in the form of a photon, which is a component of the X-ray flux.

The tube where the device is placed is surrounded on all sides by a lead sheath, which prevents the chaotic emission of photons in all directions. There is a single slit in the shell that sets the direction of movement of the X-rays. At a certain distance from the tube, a camera is placed that captures photons, and between the camera and the tube a patient (his arm, leg, etc.) is placed, who needs to be examined. Thus, some photons will be absorbed by bones and dense tissues, and some will fly through soft tissues and hit the camera. The silhouette formed on the screen will give a picture of the internal structure of the body.
Despite all the positive aspects of X-rays, they have a significant negative factor. In the early days of using X-ray machines, doctors exposed patients to radiation that was prohibitive in duration and power, which ultimately led to the development of radiation sickness in both. This is because X-rays are a form of ionizing radiation. Under its influence, some electrons are knocked out from the outer shells of atoms, which leads to ionization of the material they form. This, in turn, can lead to the destruction of soft tissue cells, which can subsequently lead to cancer, infertility, mutations and other extremely negative consequences.
However, it’s not worth it, they are afraid of x-ray radiation. Modern X-ray machines use very small portions of rays. If you do not conduct such an examination too often, the negative effect will be extremely small. Therefore, nowadays in almost every hospital you can find an X-ray room, without which it is difficult to imagine the treatment of many diseases and injuries.
X-rays are a type of high-energy electromagnetic radiation. It is actively used in various branches of medicine.
X-rays are electromagnetic waves whose photon energy on the electromagnetic wave scale is between ultraviolet radiation and gamma radiation (from ~10 eV to ~1 MeV), which corresponds to wavelengths from ~10^3 to ~10^−2 angstroms ( from ~10^−7 to ~10^−12 m). That is, it is incomparably harder radiation than visible light, which is on this scale between ultraviolet and infrared (“thermal”) rays.
The boundary between X-rays and gamma radiation is distinguished conditionally: their ranges intersect, gamma rays can have an energy of 1 keV. They differ in origin: gamma rays are emitted during processes occurring in atomic nuclei, while x-rays are emitted during processes involving electrons (both free and those located in the electron shells of atoms). At the same time, it is impossible to determine from the photon itself during what process it arose, that is, the division into the X-ray and gamma ranges is largely arbitrary.
The X-ray range is divided into “soft X-ray” and “hard”. The boundary between them lies at a wavelength of 2 angstroms and 6 keV of energy.
An X-ray generator is a tube in which a vacuum is created. There are electrodes located there - a cathode, to which a negative charge is applied, and a positively charged anode. The voltage between them is tens to hundreds of kilovolts. The generation of X-ray photons occurs when electrons “break off” from the cathode and crash into the surface of the anode at high speed. The resulting X-ray radiation is called “bremsstrahlung”; its photons have different wavelengths.

At the same time, photons of the characteristic spectrum are generated. Some of the electrons in the atoms of the anode substance are excited, that is, they move to higher orbits, and then return to their normal state, emitting photons of a certain wavelength. In a standard generator, both types of X-ray radiation are produced.
History of discovery
On November 8, 1895, the German scientist Wilhelm Conrad Roentgen discovered that certain substances began to glow when exposed to “cathode rays,” that is, a stream of electrons generated by a cathode ray tube. He explained this phenomenon by the influence of certain X-rays - this is how this radiation is now called in many languages. Later V.K. Roentgen studied the phenomenon he discovered. On December 22, 1895, he gave a report on this topic at the University of Würzburg.
Later it turned out that X-ray radiation had been observed earlier, but then the phenomena associated with it were not given much importance. The cathode ray tube was invented a long time ago, but before V.K. Nobody paid much attention to the X-rays about the blackening of photographic plates near it, etc. phenomena. The danger posed by penetrating radiation was also unknown.
Types and their effects on the body
“X-ray” is the mildest type of penetrating radiation. Excessive exposure to soft x-rays resembles the effects of ultraviolet radiation, but in a more severe form. A burn forms on the skin, but the damage is deeper and it heals much more slowly.
Hard X-ray is a full-fledged ionizing radiation that can lead to radiation sickness. X-ray quanta can break apart the protein molecules that make up the tissues of the human body, as well as the DNA molecules of the genome. But even if the X-ray quantum breaks up a water molecule, it makes no difference: in this case, chemically active free radicals H and OH are formed, which themselves are capable of affecting proteins and DNA. Radiation sickness occurs in a more severe form, the more the hematopoietic organs are affected.
X-rays have mutagenic and carcinogenic activity. This means that the likelihood of spontaneous mutations in cells during irradiation increases, and sometimes healthy cells can degenerate into cancerous ones. An increased likelihood of malignant tumors is a standard consequence of any radiation exposure, including X-rays. X-rays are the least dangerous type of penetrating radiation, but they can still be dangerous.
X-ray radiation: application and how it works
X-ray radiation is used in medicine, as well as in other areas of human activity.
Fluoroscopy and computed tomography
The most common use of X-rays is fluoroscopy. “X-raying” of the human body allows you to obtain a detailed image of both bones (they are visible most clearly) and images of internal organs.

The different transparency of body tissues in X-rays is associated with their chemical composition. The structural features of bones are that they contain a lot of calcium and phosphorus. Other tissues consist mainly of carbon, hydrogen, oxygen and nitrogen. A phosphorus atom weighs almost twice as much as an oxygen atom, and a calcium atom by 2.5 times (carbon, nitrogen and hydrogen are even lighter than oxygen). In this regard, the absorption of X-ray photons in bones is much higher.
In addition to two-dimensional “snapshots,” radiography makes it possible to create a three-dimensional image of an organ: this type of radiography is called computed tomography. For these purposes, soft x-rays are used. The amount of radiation received from one image is small: it is approximately equal to the radiation received during a 2-hour flight in an airplane at an altitude of 10 km.
X-ray flaw detection allows you to detect minor internal defects in products. It uses hard X-rays, since many materials (metal, for example) are poorly “transparent” due to the high atomic mass of their constituent substance.

X-ray diffraction and X-ray fluorescence analysis
X-rays have properties that allow them to examine individual atoms in detail. X-ray diffraction analysis is actively used in chemistry (including biochemistry) and crystallography. The principle of its operation is diffraction scattering of X-rays on atoms of crystals or complex molecules. Using X-ray diffraction analysis, the structure of the DNA molecule was determined.

X-ray fluorescence analysis allows you to quickly determine the chemical composition of a substance.
There are many forms of radiotherapy, but they all involve the use of ionizing radiation. Radiotherapy is divided into 2 types: corpuscular and wave. Corpuscular uses fluxes of alpha particles (nuclei of helium atoms), beta particles (electrons), neutrons, protons, and heavy ions. Wave uses rays of the electromagnetic spectrum - X-rays and gamma.
Radiotherapy methods are used primarily for the treatment of cancer. The fact is that radiation primarily affects actively dividing cells, which is why the hematopoietic organs suffer so much (their cells are constantly dividing, producing more and more new red blood cells). Cancer cells also constantly divide and are more vulnerable to radiation than healthy tissue.

A level of radiation is used that suppresses the activity of cancer cells while having a moderate effect on healthy cells. Under the influence of radiation, it is not the destruction of cells as such that occurs, but the damage to their genome - DNA molecules. A cell with a destroyed genome can exist for some time, but can no longer divide, that is, tumor growth stops.
X-ray therapy is the mildest form of radiotherapy. Wave radiation is softer than corpuscular radiation, and x-rays are softer than gamma radiation.
During pregnancy
Using ionizing radiation during pregnancy is dangerous. X-rays are mutagenic and can cause problems in the fetus. X-ray therapy is incompatible with pregnancy: it can only be used if it has already been decided to have an abortion. The restrictions on fluoroscopy are milder, but in the first months it is also strictly prohibited.
If absolutely necessary, X-ray examination is replaced by magnetic resonance imaging. But in the first trimester they try to avoid it too (this method appeared recently, and we can say with absolute certainty that there are no harmful consequences).

A clear danger arises when exposed to a total dose of at least 1 mSv (in old units - 100 mR). With a simple x-ray (for example, when undergoing fluorography), the patient receives approximately 50 times less. In order to receive such a dose at one time, you need to undergo a detailed computed tomography.
That is, the fact of a 1-2 x “X-ray” in itself at an early stage of pregnancy does not threaten serious consequences (but it is better not to risk it).
Treatment with it
X-rays are used primarily in the fight against malignant tumors. This method is good because it is highly effective: it kills the tumor. It is bad in that healthy tissues fare little better and there are numerous side effects. The hematopoietic organs are in particular danger.
In practice, various methods are used to reduce the impact of x-rays on healthy tissue. The rays are directed at an angle so that the tumor is in the area of their intersection (due to this, the main absorption of energy occurs right there). Sometimes the procedure is performed in motion: the patient’s body rotates relative to the radiation source around an axis passing through the tumor. In this case, healthy tissues are in the irradiation zone only occasionally, and sick tissues are constantly exposed.
X-rays are used in the treatment of certain arthrosis and similar diseases, as well as skin diseases. In this case, the pain syndrome is reduced by 50-90%. Since the radiation used is softer, side effects similar to those that occur in the treatment of tumors are not observed.