Ethanol is a primary alcohol. Alcohols - concept, properties, application
Along with hydrocarbons C A N V, which contain two types of atoms - C and H, oxygen-containing organic compounds of type C are known A N V ABOUT With. In Topic 2 we will look at oxygen-containing compounds that differ:
1) the number of O atoms in the molecule (one, two or more);
2) the multiplicity of the carbon–oxygen bond (single C–O or double C=O);
3) the type of atoms connected to oxygen (C–O–H and C–O–C).
Lesson 16.
Monohydric saturated alcohols
Alcohols are derivatives of hydrocarbons with the general formula ROH, where R is a hydrocarbon radical. The formula of an alcohol is obtained from the formula of the corresponding alkane by replacing the H atom with an OH group: RH ROH.
The chemical formula of alcohols can be derived differently, including the oxygen atom O between the atoms
C–H of a hydrocarbon molecule:
RH ROH, CH 3 –H CH 3 –O–H.
The hydroxyl group OH is alcohol functional group. That is, the OH group is a feature of alcohols; it determines the main physical and chemical properties of these compounds.
The general formula of monohydric saturated alcohols is C n H 2 n+1OH.
Names of alcohols obtained from the names of hydrocarbons with the same number of C atoms as in alcohol by adding the suffix - ol-. For example:
![]()
The name alcohols as derivatives of the corresponding alkanes is characteristic of compounds with a linear chain. The position of the OH group in them is at the outer or inner atom
C – indicated with a number after the name:
The names of alcohols - derivatives of branched hydrocarbons - are compiled in the usual way. Select the main carbon chain, which must include a C atom connected to an OH group. The C atoms of the main chain are numbered so that the carbon with the OH group receives a lower number:

The name is compiled starting with a number indicating the position of the substituent in the main carbon chain: “3-methyl...” Then the main chain is named: “3-methylbutane...” Finally, the suffix is added - ol-(name of the OH group) and the number indicates the carbon atom to which the OH group is bonded: “3-methylbutanol-2.”
If there are several substituents on the main chain, they are listed sequentially, indicating the position of each with a number. Repeating substituents in the name are written using the prefixes “di-,” “tri-,” “tetra-,” etc. For example:

Isomerism of alcohols. Alcohol isomers have the same molecular formula, but a different order of connection of atoms in the molecules.
Two types of isomerism of alcohols:
1) carbon skeleton isomerism;
2)isomerism of the position of the hydroxyl group in the molecule.
Let us present the alcohol isomers C 5 H 11 OH of these two types in linear-angular notation:

According to the number of C atoms bonded to the alcohol (–C–OH) carbon, i.e. neighboring alcohols are called primary(one neighbor C), secondary(two C) and tertiary(three C-substituents at carbon –C–OH). For example:

Task. Compose one isomer of alcohols with the molecular formula C 6 H 13 OH with a main carbon chain:
a) C 6, b) C 5, V) C 4, G) C 3
and name them.
Solution
1) We write down the main carbon chains with a given number of C atoms, leaving space for H atoms (we will indicate them later):
a) С–С–С–С–С–С; b) С–С–С–С–С; c) S–S–S–S; d) S–S–S.
2) We arbitrarily select the place of attachment of the OH group to the main chain and indicate carbon substituents at the internal C atoms:
In example d) it is not possible to place three CH 3 substituents at the C-2 atom of the main chain. Alcohol C 6 H 13 OH does not have isomers with a three-carbon main chain.
3) We arrange the H atoms at the carbons of the main chain of isomers a)–c), guided by the valence of carbon C(IV), and name the compounds:
EXERCISES.
1. Underline the chemical formulas of saturated monohydric alcohols:
CH 3 OH, C 2 H 5 OH, CH 2 = CH CH 2 OH, CH CH 2 OH, C 3 H 7 OH,
CH 3 CHO, C 6 H 5 CH 2 OH, C 4 H 9 OH, C 2 H 5 OC 2 H 5, HOCH 2 CH 2 OH.
2. Name the following alcohols:

3.
Make up structural formulas based on the names of alcohols: a) hexanol-3;
b) 2-methylpentanol-2; c) n-octanol; d) 1-phenylpropanol-1; e) 1-cyclohexylethanol.
4.
Compose the structural formulas of isomers of alcohols with the general formula C 6 H 13 OH :
a) primary; b) secondary; c) tertiary.Name these alcohols.
5. Using the linear-angular (graphical) formulas of the compounds, write down their structural formulas and give names to the substances:
Lesson 17. Preparation of alcohols
Low molecular alcohols - methanol CH 3 OH, ethanol C 2 H 5 OH, propanol C 3 H 7 OH, and isopropanol (CH 3) 2 CHOH - are colorless mobile liquids with a specific alcoholic odor. High boiling points: 64.7 °C – CH 3 OH, 78 °C – C 2 H 5 OH, 97 °C – n-C 3 H 7 OH and 82 °C – (CH 3) 2 CHOH – are due to intermolecular hydrogen bond, existing in alcohols. Alcohols C (1) – C (3) are mixed with water (dissolved) in any ratio. These alcohols, especially methanol and ethanol, are the most widely used in industry.
1. Methanol synthesized from water gas:

2. Ethanol get ethylene hydration(by adding water to C 2 H 4):
3. Another way to receive ethanol – fermentation of sugary substances under the action of yeast enzymes. The process of alcoholic fermentation of glucose (grape sugar) has the form:
4. Ethanol get from starch, and made of wood(cellulose) by hydrolysis to glucose and subsequent fermentation into alcohol:
5. Higher alcohols get from halogenated hydrocarbons by hydrolysis under the influence of aqueous solutions of alkalis:
Task.How to get 1-propanol from propane?
Solution
Of the five methods for producing alcohols proposed above, none of them considers the production of alcohol from an alkane (propane, etc.). Therefore, the synthesis of 1-propanol from propane will include several stages. According to method 2, alcohols are obtained from alkenes, which in turn are available by dehydrogenation of alkanes. The process diagram is as follows:
Another scheme for the same synthesis is one step longer, but it is easier to implement in the laboratory:
The addition of water to propene at the last stage proceeds according to Markovnikov’s rule and leads to a secondary alcohol - propanol-2. The task requires you to obtain 1-propanol. Therefore, the problem is not solved, we are looking for another way.
Method 5 consists of hydrolysis of haloalkanes. The necessary intermediate for the synthesis of 1-propanol, 1-chloropropane, is obtained as follows. Chlorination of propane gives a mixture of 1- and 2-monochloropropanes:

1-chloropropane is isolated from this mixture (for example, using gas chromatography or due to different boiling points: for 1-chloropropane t kip = 47 °C, for 2-chloropropane t kip = 36 °C). By treating 1-chloropropane with aqueous alkali KOH or NaOH, the target propanol-1 is synthesized:
Please note that the interaction of the same substances: CH 3 CH 2 CH 2 Cl and KOH - depending on the solvent (alcohol C 2 H 5 OH or water) leads to different products - propylene
(in alcohol) or propanol-1 (in water).
EXERCISES.
1. Give reaction equations for the industrial synthesis of methanol from water gas and ethanol by ethylene hydration.
2. Primary alcohols RCH 2 OH prepared by hydrolysis of primary alkyl halides RCH 2 Hal, and secondary alcohols are synthesized by hydration of alkenes. Complete the reaction equations:

3.
Suggest methods for producing alcohols: a) butanol-1; b) butanol-2;
c) pentanol-3, starting from alkenes and alkyl halides.
4. During enzymatic fermentation of sugars, along with ethanol, a mixture of primary alcohols is formed in small quantities C 3 – C 5 – fusel oil. The main component in this mixture is isopentanol.(CH 3) 2 CHCH 2 CH 2 OH, minor components – n-C 3 H 7 OH, (CH 3) 2 CHCH 2 OH and CH 3 CH 2 CH(CH 3)CH 2 OH. Name these “fusel” alcohols according to the IUPAC nomenclature. Write an equation for the fermentation reaction of glucose C 6 H 12 O 6, in which all four impurity alcohols would be obtained in a molar ratio of 2:1:1:1, respectively. Enter gas CO 2 to the right side of the equation in the amount of 1/3 mol of all initial atoms WITH , as well as the required number of molecules H 2 O.
5.
Give the formulas of all aromatic alcohols of the composition C 8 H 10 O. (In aromatic alcohols the group HE removed from the benzene ring by one or more atoms WITH:
C 6 H 5 –
(CH 2)n –
HE.)
Answers to exercises for topic 2
Lesson 16
1. The chemical formulas of saturated monohydric alcohols are underlined:
CH 3 HE, WITH 2 N 5 HE, CH 2 = CHCH 2 OH, CHCH 2 OH, WITH 3 N 7 HE,
CH 3 CHO, C 6 H 5 CH 2 OH, WITH 4 N 9 HE, C 2 H 5 OS 2 H 5 , HOCH 2 CH 2 OH.
2. Names of alcohols by structural formulas:

3. Structural formulas by alcohol names:
4. Isomers and names of alcohols of the general formula C 6 H 13 OH:

5. Structural formulas and names compiled from graphical connection diagrams:
(alcohols) a class of organic compounds containing one or more COH groups, with the hydroxyl group OH bonded to an aliphatic carbon atom (compounds in which the carbon atom in the COH group is part of the aromatic ring are called phenols)The classification of alcohols is varied and depends on which structural feature is taken as a basis.
1. Depending on the number of hydroxyl groups in the molecule, alcohols are divided into:
a) monoatomic (contain one hydroxyl OH group), for example, methanol CH 3 OH, ethanol C 2 H 5 OH, propanol C 3 H 7 OH
b) polyatomic (two or more hydroxyl groups), for example, ethylene glycol
HO С H 2 CH 2 OH , glycerol HOCH 2 CH(OH)CH 2 OH, pentaerythritol C(CH 2 OH) 4.Compounds in which one carbon atom
There are two hydroxyl groups, in most cases they are unstable and easily turn into aldehydes, eliminating water: RCH (OH) 2 ® RCH = O + H 2 O , does not exist.2. Based on the type of carbon atom to which the OH group is bonded, alcohols are divided into:
a) primary, in which the OH group is bonded to the primary carbon atom. A carbon atom (highlighted in red) that is bonded to just one carbon atom is called primary. Examples of primary alcohols ethanol C
H 3 CH 2 OH, propanol C H 3 CH 2 CH 2 OH. b) secondary, in which the OH group is bonded to a secondary carbon atom. A secondary carbon atom (highlighted in blue) is bonded to two carbon atoms at the same time, for example, secondary propanol, secondary butanol (Fig. 1).
Rice. 1. STRUCTURE OF SECONDARY ALCOHOLS
c) tertiary, in which the OH group is bonded to the tertiary carbon atom. The tertiary carbon atom (highlighted in green) is bonded to three neighboring carbon atoms simultaneously, for example, tertiary butanol and pentanol (Figure 2).

Rice. 2. STRUCTURE OF TERTIARY ALCOHOLS
According to the type of carbon atom, the alcohol group attached to it is also called primary, secondary or tertiary.
In polyhydric alcohols containing two or more OH groups, both primary and secondary HO groups may be present simultaneously, for example, in glycerol or xylitol (Fig. 3).

Rice. 3. COMBINATION OF PRIMARY AND SECONDARY OH-GROUPS IN THE STRUCTURE OF POLYATOMIC ALCOHOLS.
3. According to the structure of organic groups connected by an OH group, alcohols are divided into saturated (methanol, ethanol, propanol), unsaturated, for example, allyl alcohol CH 2 = CHCH 2 OH, aromatic (for example, benzyl alcohol C 6 H 5 CH 2 OH), containing as part of the group
R aromatic group.Unsaturated alcohols in which the OH group is “adjacent” to the double bond, i.e. bonded to a carbon atom simultaneously involved in the formation of a double bond (for example, vinyl alcohol CH 2 =CHOH), are extremely unstable and immediately isomerize ( cm ISOMERIZATION) to aldehydes or ketones:
CH 2 =CHOH ® CH 3 CH=O Nomenclature of alcohols. For common alcohols with a simple structure, a simplified nomenclature is used: the name of the organic group is converted into an adjective (using the suffix and ending “ new") and add the word "alcohol":In the case where the structure of an organic group is more complex, rules common to all organic chemistry are used. Names compiled according to such rules are called systematic. In accordance with these rules, the hydrocarbon chain is numbered from the end to which the OH group is located closest. Next, this numbering is used to indicate the position of various substituents along the main chain; at the end of the name, the suffix “ol” and a number indicating the position of the OH group are added (Fig. 4): 4. SYSTEMATIC NAMES OF ALCOHOLS. Functional (OH) and substituent (CH 3) groups, as well as their corresponding digital indices, are highlighted in different colors.The systematic names of the simplest alcohols follow the same rules: methanol, ethanol, butanol. For some alcohols, trivial (simplified) names that developed historically have been preserved: propargyl alcohol NSє
CCH 2 OH, glycerol HOCH 2 CH(OH)CH 2 OH, pentaerythritol C(CH 2 OH) 4, phenethyl alcohol C 6 H 5 CH 2 CH 2 OH.Physical properties of alcohols.
Alcohols are soluble in most organic solvents; the first three simplest representatives - methanol, ethanol and propanol, as well as tertiary butanol (H 3 C) 3 СОН are mixed with water in any ratio. With an increase in the number of C atoms in the organic group, the hydrophobic (water-repellent) effect begins to affect, solubility in water becomes limited, and when R containing more than 9 carbon atoms practically disappears.
4. SYSTEMATIC NAMES OF ALCOHOLS. Functional (OH) and substituent (CH 3) groups, as well as their corresponding digital indices, are highlighted in different colors.The systematic names of the simplest alcohols follow the same rules: methanol, ethanol, butanol. For some alcohols, trivial (simplified) names that developed historically have been preserved: propargyl alcohol NSє
CCH 2 OH, glycerol HOCH 2 CH(OH)CH 2 OH, pentaerythritol C(CH 2 OH) 4, phenethyl alcohol C 6 H 5 CH 2 CH 2 OH.Physical properties of alcohols.
Alcohols are soluble in most organic solvents; the first three simplest representatives - methanol, ethanol and propanol, as well as tertiary butanol (H 3 C) 3 СОН are mixed with water in any ratio. With an increase in the number of C atoms in the organic group, the hydrophobic (water-repellent) effect begins to affect, solubility in water becomes limited, and when R containing more than 9 carbon atoms practically disappears. Due to the presence of OH groups, hydrogen bonds arise between alcohol molecules.
Rice. 5. HYDROGEN BONDS IN ALCOHOLS(shown in dotted line)
As a result, all alcohols have a higher boiling point than the corresponding hydrocarbons, e.g. bp. ethanol +78° C, and T. boil. ethane 88.63° C; T. kip. butanol and butane, respectively, +117.4° C and 0.5° C.
Chemical properties of alcohols. Alcohols have a variety of transformations. The reactions of alcohols have some general principles: the reactivity of primary monohydric alcohols is higher than secondary ones, in turn, secondary alcohols are chemically more active than tertiary ones. For dihydric alcohols, in the case when OH groups are located at neighboring carbon atoms, increased (compared to monohydric alcohols) reactivity is observed due to the mutual influence of these groups. For alcohols, reactions are possible that involve the breaking of both CO and OH bonds.1. Reactions occurring through the OH bond.
When interacting with active metals (Na, K, Mg, Al), alcohols exhibit the properties of weak acids and form salts called alcoholates or alkoxides:
CH 3 OH + 2 Na ® 2 CH 3 OK + H 2Alcoholates are chemically unstable and, when exposed to water, hydrolyze to form alcohol and metal hydroxide:
C 2 H 5 OK + H 2 O
® C 2 H 5 OH + KOHThis reaction shows that alcohols are weaker acids compared to water (a strong acid displaces a weak one); in addition, when interacting with alkali solutions, alcohols do not form alcoholates. However, in polyhydric alcohols (in the case when OH groups are attached to neighboring C atoms), the acidity of the alcohol groups is much higher, and they can form alcoholates not only when interacting with metals, but also with alkalis:
HOCH 2 CH 2 OH + 2NaOH ® NaOCH 2 CH 2 ONa + 2H 2 OWhen HO groups in polyhydric alcohols are attached to non-adjacent C atoms, the properties of alcohols are close to monoatomic ones, since the mutual influence of HO groups does not appear.When interacting with mineral or organic acids, alcohols form esters compounds containing a fragment
ROA (A acid residue). The formation of esters also occurs during the interaction of alcohols with anhydrides and acid chlorides carboxylic acids(Fig. 6).Under the action of oxidizing agents (K 2 Cr 2 O 7, KMnO 4), primary alcohols form aldehydes, and secondary alcohols form ketones (Fig. 7)

Rice. 7. FORMATION OF ALDEHYDES AND KETONES DURING THE OXIDATION OF ALCOHOLS
The reduction of alcohols leads to the formation of hydrocarbons containing the same number of C atoms as the molecule of the original alcohol (Fig. 8).
 8. BUTANOL RESTORATION
8. BUTANOL RESTORATION2. Reactions occurring through the CO bond.
In the presence of catalysts or strong mineral acids, dehydration of alcohols (elimination of water) occurs, and the reaction can proceed in two directions:
a) intermolecular dehydration involving two alcohol molecules, in which the CO bonds in one of the molecules are broken, resulting in the formation of ethers - compounds containing a fragment
R О R (Fig. 9A).b) intramolecular dehydration produces alkenes - hydrocarbons with a double bond. Often both processes, the formation of an ether and an alkene, occur in parallel (Fig. 9B).
In the case of secondary alcohols, during the formation of an alkene, two reaction directions are possible (Fig. 9B), the predominant direction is one in which, during the condensation process, hydrogen is split off from the least hydrogenated carbon atom (marked by number 3), i.e. surrounded by fewer hydrogen atoms (compared to atom 1). Shown in Fig. 10 reactions are used to produce alkenes and ethers.
The cleavage of the CO bond in alcohols also occurs when the OH group is replaced by a halogen or amino group (Fig. 10).

Rice. 10. REPLACEMENT OF OH-GROUP IN ALCOHOLS WITH HALOGEN OR AMINO GROUP
The reactions shown in Fig. 10 is used for the production of halocarbons and amines.
Preparation of alcohols. Some of the reactions shown above (Fig. 6,9,10) are reversible and, when conditions change, can proceed in the opposite direction, leading to the production of alcohols, for example, during the hydrolysis of esters and halocarbons (Fig. 11A and B, respectively), as well as by hydration alkenes by adding water (Fig. 11B).
Rice. eleven. OBTAINING ALCOHOLS BY HYDROLYSIS AND HYDRATION OF ORGANIC COMPOUNDS
The hydrolysis reaction of alkenes (Fig. 11, Scheme B) underlies the industrial production of lower alcohols containing up to 4 C atoms.
Ethanol is also formed during the so-called alcoholic fermentation of sugars, for example, glucose C 6 H 12 O 6. The process occurs in the presence of yeast and leads to the formation of ethanol and CO 2:
® 2C 2 H 5 OH + 2CO 2Fermentation can produce no more than a 15% aqueous solution of alcohol, since at a higher concentration of alcohol the yeast fungi die. Higher concentration alcohol solutions are obtained by distillation.
Methanol is produced industrially by the reduction of carbon monoxide at 400
° C under a pressure of 2030 MPa in the presence of a catalyst consisting of oxides of copper, chromium, and aluminum:® H 3 SON If instead of hydrolysis of alkenes (Fig. 11) oxidation is carried out, then dihydric alcohols are formed (Fig. 12) 12.
PREPARATION OF DIOHOMIC ALCOHOLSUse of alcohols.
The ability of alcohols to participate in a variety of chemical reactions allows them to be used to produce all kinds of organic compounds: aldehydes, ketones, carboxylic acids, ethers and esters, used as organic solvents in the production of polymers, dyes and drugs.
12.
PREPARATION OF DIOHOMIC ALCOHOLSUse of alcohols.
The ability of alcohols to participate in a variety of chemical reactions allows them to be used to produce all kinds of organic compounds: aldehydes, ketones, carboxylic acids, ethers and esters, used as organic solvents in the production of polymers, dyes and drugs. Methanol CH 3 OH is used as a solvent, as well as in the production of formaldehyde, used to produce phenol-formaldehyde resins; methanol has recently been considered as a promising motor fuel. Large volumes of methanol are used in the production and transportation of natural gas. Methanol the most toxic compound among all alcohols, lethal dose when taken orally 100 ml.
Ethanol C 2 H 5 OH the starting compound for the production of acetaldehyde, acetic acid, as well as for the production of esters of carboxylic acids used as solvents. In addition, ethanol is the main component of all alcoholic beverages; it is widely used in medicine as a disinfectant.
Butanol is used as a solvent for fats and resins; in addition, it serves as a raw material for the production of fragrant substances (butyl acetate, butyl salicylate, etc.). In shampoos it is used as a component that increases the transparency of solutions.
Benzyl alcohol C 6 H 5 CH 2 OH in the free state (and in the form of esters) is found in the essential oils of jasmine and hyacinth. It has antiseptic (disinfecting) properties; in cosmetics it is used as a preservative for creams, lotions, dental elixirs, and in perfumery as a fragrant substance.
Phenethyl alcohol C 6 H 5 CH 2 CH 2 OH has a rose scent, is found in rose oil, and is used in perfumery.
Ethylene glycol HOCH 2 CH 2 OH is used in the production of plastics and as an antifreeze (an additive that reduces the freezing point of aqueous solutions), in addition, in the manufacture of textile and printing inks.
Diethylene glycol HOCH 2 CH 2 OCH 2 CH 2 OH is used to fill hydraulic brake devices, as well as in the textile industry for finishing and dyeing fabrics.
Glycerol
HOCH 2 CH (OH ) CH 2 OH It is used to produce polyester glyphthalic resins; in addition, it is a component of many cosmetic preparations. Nitroglycerin (Fig. 6) is the main component of dynamite, used in mining and railway construction as an explosive.Pentaerythritol (
HOCH 2) 4 C is used to produce polyesters (pentaphthalic resins), as a hardener for synthetic resins, as a plasticizer for polyvinyl chloride, and also in the production of the explosive tetranitropentaerythritol.Polyhydric alcohols xylitol HOCH 2 (CHOH) 3 CH 2 OH and sorbitol neHOCH 2 (CHOH) 4 CH 2 OH have a sweet taste, they are used instead of sugar in the production of confectionery products for patients with diabetes and people suffering from obesity. Sorbitol is found in rowan and cherry berries.
Mikhail Levitsky
LITERATURE Shabarov Yu.S. Organic chemistry. Moscow, “Chemistry”, 1994Chemical properties of monohydric saturated alcohols.
I. Substitution reactions
1. Substitution of hydrogen atoms of the hydroxyl group due to the cleavage of the O–H bond
The rate of reactions in which the O–H bond is broken decreases in the series: primary alcohols → secondary → tertiary.
a) Interaction with active metals to form metal alkagolates (alkanolates):
2C 2 H 5 −OH + 2Na → C 2 H 5 −ONa + H 2
Alcoholates They are similar to salts of a very weak acid, and they are also easily hydrolyzed. Alcoholates are extremely unstable and when exposed to water, they decompose into alcohol and alkali. This proves that alcohols are weaker acids than water. From this it follows that monohydric alcohols do not react with alkalis!
C 2 H 5 −ONa + HOH → C 2 H 5 −OH + NaOH
b) Interaction with organic and inorganic acids to form esters ( esterification reaction)
C 2 H 5 −OH + HO−NO 2 ↔ C 2 H 5 −O−NO 2 + HOH
Nitric acid ethyl ester
CH 3 −COOH + HO−C 2 H 5 ↔ CH 3 COO−C 2 H 5 + HOH
Ethyl acetic acid
2. Substitution of hydroxyl group due to cleavage of the C–O bond
a) Alcohol solutions have a neutral reaction to indicators.
b) Reaction with ammonia to form primary amines (and with an excess of alcohol, alkyl radicals can replace 2 or 3 hydrogen atoms in NH3 and form secondary and tertiary amines)
C 2 H 5 −OH + H−NH 2 → C 2 H 5 − NH 2 + H−OH.
Ethylamine
C 2 H 5 −OH + H−NH−C 2 H 5 → NH−(C 2 H 5) 2 + H−OH.
Diethylamine
c) Reaction with hydrogen halides to form haloalkanes
C 2 H 5 −OH + HCl → C 2 H 5 −Cl + HOH.
d) Reaction with thionyl chloride to form haloalkanes
C 4 H 9 −OH + SO 2 Cl 2 → C 4 H 9 −Cl + HCl + SO 2 .
e) Reaction with phosphorus chloride to form haloalkanes
C 4 H 9 −OH + PCl 5 → C 4 H 9 −Cl + POCl 3 + HCl.
II. Elimination reactions
1. Dehydration reaction, i.e. splitting off a water molecule
a) Intermolecular dehydration of alcohols with the formation of ethers R−O−R"
C 2 H 5 −OH + HO−C 2 H 5 → C 2 H 5 −O− C 2 H 5 + H−OH.
Diethyl ether
b) Intramolecular dehydration of alcohols with the formation of alkenes
H−CH 2 −CH 2 −OH → CH 2 =CH 2 + H−OH.
2. Dehydrogenation reaction (breaking O–H and C–H bonds)
a) When primary alcohols are dehydrogenated, aldehydes are formed
CH 3 −CH−O−H → CH 3 −CH=O + H 2
b) When secondary alcohols are dehydrogenated, ketones are formed
CH 3 −C−CH 3 → CH 3 −C−CH 3 + H 2
c) Tertiary alcohols do not dehydrogenate
III. Oxidation reactions
a) Combustion (complete oxidation) of alcohols
C 2 H 5 OH + 3O 2 → 2CO 2 + 3H 2 O +Q.
When they burn, a lot of heat is released, which is often used in laboratories (laboratory burners). Lower alcohols burn with an almost colorless flame, while higher alcohols have a yellowish flame due to incomplete combustion of carbon.
b) Incomplete oxidation of alcohols with atmospheric oxygen with the formation of aldehydes or with further oxidation of carboxylic acid (from primary alcohols) and ketones (from secondary alcohols)
2CH 3 OH + O 2 → 2HCH=O + 2H 2 O,
CH 3 −CH 2 OH + O 2 → CH 3 −COOH + H 2 O,
2CH 3 −CH(OH)−CH 3 + O 2 → 2CH 3 −C(=O)−CH 3 + 2H 2 O.
c) Incomplete oxidation of alcohols with oxidizing oxygen in the presence of a catalyst with the formation of aldehydes or with further oxidation of carboxylic acid (from primary alcohols) and ketones (from secondary alcohols)
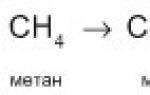
CH 4 + [O] → HCH=O + H 2 O,
CH 3 −CH 2 OH + 2[O] → CH 3 −COOH + H 2 O,
CH 3 −CH(OH)−CH 3 + [O] → CH 3 −C(=O)−CH 3 + H 2 O.
Chemical properties of polyhydric saturated alcohols
Chemical properties of polyhydric alcohols the same as monohydric alcohols, but the difference is that the reaction does not proceed one at a time to the hydroxyl group, but several at once. One of the main differences is polyhydric alcohols easily react with a freshly prepared solution of copper (II) hydroxide (blue precipitate). This produces a transparent solution of a complex copper salt of a bright blue-violet color. It is this reaction that can detect the presence of a polyhydric alcohol in any solution.
Use of alcohols.
The ability of alcohols to participate in a variety of chemical reactions allows them to be used to produce all kinds of organic compounds: aldehydes, ketones, carboxylic acids, ethers and esters, used as organic solvents in the production of polymers, dyes and drugs.
Methanol CH 3 OH used as a solvent, as well as in the production of formaldehyde, used to produce phenol-formaldehyde resins; methanol has recently been considered as a promising motor fuel. Large volumes of methanol are used in the production and transportation of natural gas. Methanol is the most toxic compound among all alcohols, the lethal dose when ingested is 100 ml.
Ethanol C 2 H 5 OH– the starting compound for the production of acetaldehyde, acetic acid, as well as for the production of esters of carboxylic acids used as solvents, medicines, perfumes and colognes, rubbers, fuel for engines, dyes, varnishes, solvents and other substances. In addition, ethanol is the main component of all alcoholic beverages; it is widely used in medicine as a disinfectant.
Butanol used as a solvent for fats and resins, in addition, it serves as a raw material for the production of fragrant substances (butyl acetate, butyl salicylate, etc.). In shampoos it is used as a component that increases the transparency of solutions.
Benzyl alcohol C 6 H 5 –CH 2 –OH in a free state (and in the form of esters) is found in essential oils of jasmine and hyacinth. It has antiseptic (disinfecting) properties; in cosmetics it is used as a preservative for creams, lotions, dental elixirs, and in perfumery as a fragrant substance.
Phenethyl alcohol C 6 H 5 –CH 2 –CH 2 –OH It has a rose scent, is found in rose oil, and is used in perfumery.
Ethylene glycol HOCH 2 –CH 2 OH used in the production of plastics and as an antifreeze (an additive that reduces the freezing point of aqueous solutions), in addition, in the manufacture of textile and printing inks. Dinitroethylene glycol used as explosives
Diethylene glycol HOCH 2 –CH 2 OCH 2 –CH 2 OH used for filling brake hydraulic devices, as well as in the textile industry for finishing and dyeing fabrics.
Glycerol HOCH 2 –CH(OH)–CH 2 OH used to produce polyester glyphthalic resins; in addition, it is a component of many cosmetic preparations as a preservative and as a means of preventing freezing and drying! Nitroglycerine used as explosives - as the main component of dynamite, used in mining and railway construction as an explosive. Trinitroglycerin− also in medicine, as a vasodilator.
Pentaerythritol (HOCH 2) 4 C used to produce polyesters (pentaphthalic resins), as a hardener for synthetic resins, as a plasticizer for polyvinyl chloride, and also in the production of the explosive tetranitropentaerythritol.
Polyhydric alcohols xylitol HOCH2–(CHOH)3–CH2OH And sorbitol СОН2– (СНН)4–СН2ОН They have a sweet taste and are used instead of sugar in the production of confectionery products for diabetics and people suffering from obesity. Sorbitol is found in rowan and cherry berries.
Questions: (to control knowledge)
- What substances belong to alcohols and how are the names of their compounds formed?
- What types of isomerism are characteristic of alcohols? Give examples.
- By what reactions can alcohols be obtained?
- What chemical reactions are characteristic of saturated alcohols? Give reaction equations.
- Where are alcohols used?
List of sources used.
Intoxicating drinks, which contain ethanol - monohydric wine alcohol, have been familiar to mankind since ancient times. They were made from honey and fermented fruits. In ancient China, rice was also added to drinks.
Alcohol from wine was obtained in the East (VI-VII centuries). European scientists created it from fermentation products in the 11th century. The Russian royal court became acquainted with it in the 14th century: the Genoese embassy presented it as living water (“aqua vita”).
THOSE. Lovitz, a Russian scientist of the 18th century, was the first to experimentally obtain absolute ethyl alcohol by distillation using potash - potassium carbonate. The chemist suggested using charcoal for cleaning.
Thanks to the scientific achievements of the 19th and 20th centuries. The global use of alcohols became possible. Scientists of the past developed a theory of the structure of aqueous-alcohol solutions and studied their physicochemical properties. Fermentation methods were discovered: cyclic and continuous flow.
Significant inventions of chemical science of the past, which made the beneficial properties of alcohols real:
- Barbe ratification apparatus (1881)
- Saval's distillation plate apparatus (1813)
- Gentse's boiler (1873)
A homologous series of alcoholic substances was discovered. A series of experiments on the synthesis of methanol and ethylene glycol were carried out. Advanced scientific research in the post-war years of the 20th century helped improve the quality of products. We raised the level of the domestic alcohol industry.
Distribution in nature
In nature, alcohols occur in free form. The substances are also components of esters. The natural fermentation process of carbohydrate-containing foods creates ethanol, as well as 1-butanol and isopropanol. Alcohols in the baking industry, brewing, and winemaking are associated with the use of the fermentation process in these industries. Most insect pheromones are alcohols.
Alcohol derivatives of carbohydrates in nature:
- sorbitol - found in rowan and cherry berries, has a sweet taste.
Many plant aromatic substances are terpene alcohols:
- fenhol - a component of fennel fruits, coniferous tree resins
- borneol - a constituent element of the wood of the borneocamphor tree
- menthol is a component of geranium and mint composition
Bile of humans and animals contains bile polyhydric alcohols:
- mixinol
- chimerol
- bufol
- cholestanpentol
Harmful effects on the body
The widespread use of alcohols in agriculture, industry, military affairs, and transport makes them accessible to ordinary citizens. This causes acute, including mass, poisonings and deaths.
The dangers of methanol
Methanol is a dangerous poison. It has a toxic effect on the heart and nervous system. Ingestion of 30 g of methanol leads to death. Ingestion of a smaller amount of the substance causes severe poisoning with irreversible consequences (blindness).
Its maximum permissible concentration in the air at work is 5 mg/m³. Liquids containing even a minimal amount of methanol are dangerous.
In mild forms of poisoning, symptoms appear:
- chills
- general weakness
- nausea
- headache
Methanol tastes and smells no different from ethanol. This causes the poison to be ingested by mistake. How to distinguish ethanol from methanol at home?

Copper wire is rolled into a spiral and heated strongly over a fire. When it interacts with ethanol, the smell of rotten apples is felt. Contact with methanol will trigger an oxidation reaction. Formaldehyde will be released - a gas with an unpleasant, pungent odor.
Ethanol toxicity
Ethanol acquires toxic and narcotic properties depending on the dose, route of entry into the body, concentration, and duration of exposure.
Ethanol can cause:
- disruption of the central nervous system
- cancer of the esophagus, stomach
- gastritis
- cirrhosis of the liver
- heart diseases
4-12 g of ethanol per 1 kg of body weight is a lethal single dose. Acetaldehyde, the main metabolite of ethanol, is a carcinogenic, mutagenic, toxic substance. It changes cell membranes, the structural characteristics of red blood cells, and damages DNA. Isopropanol is similar to ethanol in toxic effects.
The production of alcohols and their circulation are regulated by the state. Ethanol is not legally recognized as a drug. But its toxic effects on the body have been proven.
The effect on the brain is especially destructive. Its volume decreases. Organic changes occur in the neurons of the cerebral cortex, their damage and death. Capillary ruptures occur.
The normal functioning of the stomach, liver, and intestines is disrupted. Excessive consumption of strong alcohol causes acute pain and diarrhea. The mucous membrane of the gastrointestinal tract is damaged, and bile stagnates.
Inhalation effects of alcohols
The widespread use of alcohols in many industries creates a threat of their inhalation effects. Toxic effects were studied in rats. The results obtained are shown in the table.
Food industry
Ethanol is the basis of alcoholic beverages. It is obtained from sugar beets, potatoes, grapes, cereals - rye, wheat, barley, and other raw materials containing sugar or starch. During the production process, modern technologies for purifying fusel oils are used.
They are divided into:
- strong with an ethanol share of 31-70% (cognac, absinthe, rum, vodka)
- medium strength - from 9 to 30% ethanol (liqueurs, wines, liqueurs)
- low alcohol - 1.5-8% (cider, beer).
Ethanol is the raw material for natural vinegar. The product is obtained by oxidation with acetic acid bacteria. Aeration (forced saturation with air) is a necessary condition for the process.
Ethanol is not the only alcohol in the food industry. Glycerin - food additive E422 - provides the connection of immiscible liquids. It is used in the manufacture of confectionery, pasta, and bakery products. Glycerin is a component of liqueurs, giving drinks a viscosity and sweet taste.
The use of glycerin has a beneficial effect on products:
- Pasta stickiness decreases
- the consistency of sweets and creams improves
- prevents rapid staleness of bread and sagging of chocolate
- Products are baked without starch sticking
The use of alcohols as sweeteners is widespread. Mannitol, xylitol, and sorbitol are suitable for this purpose.
Perfumes and cosmetics
Water, alcohol, perfume composition (concentrate) are the main components of perfume products. They are used in different proportions. The table presents the types of perfumes and the proportions of the main components.
In the production of perfumery products, highly purified ethanol acts as a solvent for fragrant substances. When reacting with water, salts are formed which precipitate. The solution settles for several days and is filtered.
2-phenylethanol replaces natural rose oil in the perfume and cosmetics industry. The liquid has a light floral scent. Included in fantasy and flower compositions, cosmetic milks, creams, elixirs, lotions.
The main base of many care products is glycerin. It is able to attract moisture, actively moisturize the skin, and make it elastic. Dry, dehydrated skin benefits from creams, masks, and soaps with glycerin: it creates a moisture-saving film on the surface and keeps the skin soft.
There is a myth: that using alcohol in cosmetics is harmful. However, these organic compounds are stabilizers, carriers of active substances, and emulsifiers necessary for the production of products.
Alcohols (especially fatty ones) make care products creamy and soften skin and hair. Ethanol in shampoos and conditioners moisturizes, evaporates quickly after washing your hair, and makes combing and styling easier.
Medicine
Ethanol is used in medical practice as an antiseptic. It destroys microbes, prevents decomposition in open wounds, and delays painful changes in the blood.
Its drying, disinfecting, tanning properties are the reason for using it to treat the hands of medical personnel before working with a patient. During artificial ventilation, ethanol is indispensable as an antifoam. If there is a shortage of medications, it becomes a component of general anesthesia.
In case of poisoning with ethylene glycol or methanol, ethanol becomes an antidote. After taking it, the concentration of toxic substances decreases. Ethanol is used in warming compresses and when rubbing for cooling. The substance restores the body during feverish heat and colds.
Alcohols in medicines and their effects on humans are studied by the science of pharmacology. Ethanol as a solvent is used in the production of extracts and tinctures of medicinal plant materials (hawthorn, pepper, ginseng, motherwort).

These liquid medicines should only be taken after medical advice. You must strictly follow the dosage prescribed by your doctor!
Fuel
The commercial availability of methanol, butanol-1, ethanol is the reason for their use as fuel. Mixed with diesel fuel, gasoline, used as fuel in its pure form. The mixtures help reduce the toxicity of exhaust gases.
Alcohol, as an alternative source of fuel, has its disadvantages:
- substances have increased corrosive characteristics, unlike hydrocarbons
- If moisture gets into the fuel system, there will be a sharp decrease in power due to the solubility of substances in water
- there is a risk of vapor locks and deterioration of engine performance due to low boiling points of substances.
However, gas and oil resources are finite. Therefore, the use of alcohols in world practice has become an alternative to the use of conventional fuel. Their mass production is being established from industrial waste (pulp and paper, food, woodworking) - at the same time the problem of recycling is being solved.
Industrial processing of plant raw materials makes it possible to obtain environmentally friendly biofuel - bioethanol. The raw materials for it are corn (USA), sugar cane (Brazil).
The positive energy balance and renewable fuel resource make bioethanol production a popular area of the global economy.
Solvents, surfactants
In addition to the production of cosmetics, perfumes, liquid medicines, and confectionery, alcohols are also good solvents:
Alcohol as a solvent:
- in the manufacture of metal surfaces, electronic elements, photographic paper, photographic films
- when cleaning natural products: resins, oils, waxes, fats
- in the process of extraction - extracting a substance
- when creating synthetic polymeric materials (glue, varnish), paints
- in the production of medical and household aerosols.
Popular solvents are isopropanol, ethanol, methanol. Polyhydric and cyclic substances are also used: glycerin, cyclohexanol, ethylene glycol.
Surfactants are produced from higher fatty alcohols. Complete care of your car, dishes, apartments, and clothes is possible thanks to surfactants. They are part of cleaning products and detergents and are used in many sectors of the economy (see table).
| Industry | Surfactants: functions, properties |
|---|---|
| Agriculture | Included in emulsions; increase the productivity of the process of transferring nutrients to plants |
| Construction | Reduce water demand for concrete and cement mixtures; increase frost resistance and density of materials |
| Leather industry | Prevents sticking and damage to products |
| Textile industry | Remove static electricity |
| Metallurgy | Reduce friction; able to withstand high temperatures |
| Paper industry | Separate boiled pulp from ink during used paper recycling |
| Paint industry | Enables complete penetration of paint onto surfaces, including small recesses |

The use of alcohols in the food industry, medicine, the production of perfumes and cosmetics, use as fuel, solvents, and surfactants has a positive effect on the state of the country’s economy. It brings convenience to a person’s life, but requires compliance with safety precautions due to the toxicity of the substances.
Structure
Alcohols (or alkanols) are organic substances whose molecules contain one or more hydroxyl groups (-OH groups) connected to a hydrocarbon radical.
Based on the number of hydroxyl groups (atomicity), alcohols are divided into:
Monatomic
dihydric (glycols)
triatomic.
The following alcohols are distinguished by their nature:
Saturated, containing only saturated hydrocarbon radicals in the molecule
unsaturated, containing multiple (double and triple) bonds between carbon atoms in the molecule
aromatic, i.e. alcohols containing a benzene ring and a hydroxyl group in the molecule, connected to each other not directly, but through carbon atoms.
Organic substances containing hydroxyl groups in the molecule, bonded directly to the carbon atom of the benzene ring, differ significantly in chemical properties from alcohols and are therefore classified as an independent class of organic compounds - phenols. For example, hydroxybenzene phenol. We will learn more about the structure, properties and use of phenols later.
There are also polyatomic (polyatomic) ones containing more than three hydroxyl groups in the molecule. For example, the simplest hexahydric alcohol is hexaol (sorbitol).
It should be noted that alcohols containing two hydroxyl groups on one carbon atom are unstable and spontaneously decompose (subject to rearrangement of atoms) to form aldehydes and ketones: 
Unsaturated alcohols containing a hydroxyl group at the carbon atom connected by a double bond are called ecols. It is not difficult to guess that the name of this class of compounds is formed from the suffixes -en and -ol, indicating the presence of a double bond and a hydroxyl group in the molecules. Enols, as a rule, are unstable and spontaneously transform (isomerize) into carbonyl compounds - aldehydes and ketones. This reaction is reversible, the process itself is called keto-enol tautomerism. Thus, the simplest enol, vinyl alcohol, isomerizes extremely quickly into acetaldehyde.
Based on the nature of the carbon atom to which the hydroxyl group is bonded, alcohols are divided into:
Primary, in the molecules of which the hydroxyl group is bonded to the primary carbon atom
secondary, in the molecules of which the hydroxyl group is bonded to a secondary carbon atom
tertiary, in the molecules of which the hydroxyl group is bonded to a tertiary carbon atom, for example:
Nomenclature and isomerism
When naming alcohols, the (generic) suffix -ol is added to the name of the hydrocarbon corresponding to the alcohol. The numbers after the suffix indicate the position of the hydroxyl group in the main chain, and the prefixes di-, tri-, tetra-, etc. indicate their number: 
Starting from the third member of the homologous series, alcohols exhibit isomerism of the position of the functional group (propanol-1 and propanol-2), and from the fourth, isomerism of the carbon skeleton (butanol-1; 2-methylpropanol-1). They are also characterized by interclass isomerism - alcohols are isomeric to ethers.
Roda, which is part of the hydroxyl group of alcohol molecules, differs sharply from hydrogen and carbon atoms in its ability to attract and hold electron pairs. Due to this, alcohol molecules contain polar C-O and O-H bonds.
Physical properties of alcohols
Given the polarity of the O-H bond and the significant partial positive charge localized (focused) on the hydrogen atom, the hydrogen of the hydroxyl group is said to be “acidic” in nature. In this way, it differs sharply from the hydrogen atoms included in the hydrocarbon radical.
It should be noted that the oxygen atom of the hydroxyl group has a partial negative charge and two lone electron pairs, which allows alcohols to form special, so-called hydrogen bonds between molecules. Hydrogen bonds occur when a partially positively charged hydrogen atom of one alcohol molecule interacts with a partially negatively charged oxygen atom of another molecule. It is thanks to hydrogen bonds between molecules that alcohols have boiling points that are abnormally high for their molecular weight. Thus, propane with a relative molecular weight of 44 under normal conditions is a gas, and the simplest of alcohols is methanol, having a relative molecular weight of 32, under normal conditions a liquid.
The lower and middle members of a series of saturated monohydric alcohols, containing from one to eleven carbon atoms, are liquids. Higher alcohols (starting from C 12 H 25 OH) are solids at room temperature. Lower alcohols have a characteristic alcoholic odor and pungent taste; they are highly soluble in water. As the hydrocarbon radical increases, the solubility of alcohols in water decreases, and octanol no longer mixes with water.
Chemical properties
The properties of organic substances are determined by their composition and structure. Alcohols confirm the general rule. Their molecules include hydrocarbon and hydroxyl radicals, so the chemical properties of alcohols are determined by the interaction and influence of these groups on each other. The properties characteristic of this class of compounds are due to the presence of a hydroxyl group.
1. Interaction of alcohols with alkali and alkaline earth metals. To identify the effect of a hydrocarbon radical on a hydroxyl group, it is necessary to compare the properties of a substance containing a hydroxyl group and a hydrocarbon radical, on the one hand, and a substance containing a hydroxyl group and not containing a hydrocarbon radical, on the other. Such substances can be, for example, ethanol (or other alcohol) and water. The hydrogen of the hydroxyl group of alcohol molecules and water molecules is capable of being reduced by alkali and alkaline earth metals (replaced by them).
With water this interaction is much more active than with alcohol, is accompanied by a large release of heat, and can lead to an explosion. This difference is explained by the electron-donating properties of the radical closest to the hydroxyl group. Possessing the properties of an electron donor (+I-effect), the radical slightly increases the electron density on the oxygen atom, “saturates” it at its own expense, thereby reducing the polarity of the O-H bond and the “acidic” nature of the hydrogen atom of the hydroxyl group in alcohol molecules by compared to water molecules.
2. Interaction of alcohols with hydrogen halides. Substitution of a hydroxyl group with a halogen leads to the formation of haloalkanes.
For example:
C2H5OH + HBr<->C2H5Br + H2O
This reaction is reversible.
3. Intermolecular dehydration of alcohols - the splitting of a water molecule from two alcohol molecules when heated in the presence of water-removing agents.
As a result of intermolecular dehydration of alcohols, ethers are formed. Thus, when ethyl alcohol is heated with sulfuric acid to a temperature of 100 to 140 ° C, diethyl (sulfur) ether is formed.
4. The interaction of alcohols with organic and inorganic acids to form esters (esterification reaction):

The esterification reaction is catalyzed by strong inorganic acids.
For example, the interaction of ethyl alcohol and acetic acid produces ethyl acetate - ethyl acetate: 
5. Intramolecular dehydration of alcohols occurs when alcohols are heated in the presence of water-removing agents to a higher temperature than the temperature of intermolecular dehydration. As a result, alkenes are formed. This reaction is due to the presence of a hydrogen atom and a hydroxyl group at adjacent carbon atoms. An example is the reaction of producing ethene (ethylene) by heating ethanol above 140 °C in the presence of concentrated sulfuric acid.
6. Oxidation of alcohols is usually carried out with strong oxidizing agents, such as potassium dichromate or potassium permanganate in an acidic environment. In this case, the action of the oxidizing agent is directed to the carbon atom that is already bonded to the hydroxyl group. Depending on the nature of the alcohol and the reaction conditions, various products can be formed. Thus, primary alcohols are oxidized first to aldehydes and then to carboxylic acids: 
Tertiary alcohols are quite resistant to oxidation. However, under harsh conditions (strong oxidizing agent, high temperature), oxidation of tertiary alcohols is possible, which occurs with the rupture of carbon-carbon bonds closest to the hydroxyl group.
7. Dehydrogenation of alcohols. When alcohol vapor is passed at 200-300 °C over a metal catalyst, such as copper, silver or platinum, primary alcohols are converted into aldehydes, and secondary alcohols into ketones:

The presence of several hydroxyl groups in the alcohol molecule at the same time determines the specific properties of polyhydric alcohols, which are capable of forming bright blue complex compounds soluble in water when interacting with a freshly obtained precipitate of copper(II) hydroxide.
Monohydric alcohols are not able to enter into this reaction. Therefore, it is a qualitative reaction to polyhydric alcohols.
Alcoholates of alkali and alkaline earth metals undergo hydrolysis when interacting with water. For example, when sodium ethoxide is dissolved in water, a reversible reaction occurs
C2H5ONa + HON<->C2H5OH + NaOH
the balance of which is almost completely shifted to the right. This also confirms that water is superior to alcohols in its acidic properties (the “acidic” nature of the hydrogen in the hydroxyl group). Thus, the interaction of alcoholates with water can be considered as the interaction of a salt of a very weak acid (in this case, the alcohol that formed the alcoholate acts as this) with a stronger acid (water plays this role here).
Alcohols can exhibit basic properties when reacting with strong acids, forming alkyloxonium salts due to the presence of a lone electron pair on the oxygen atom of the hydroxyl group: 
The esterification reaction is reversible (the reverse reaction is ester hydrolysis), the equilibrium shifts to the right in the presence of water-removing agents.
Intramolecular dehydration of alcohols proceeds in accordance with Zaitsev's rule: when water is removed from a secondary or tertiary alcohol, a hydrogen atom is detached from the least hydrogenated carbon atom. Thus, dehydration of 2-butanol results in 2-butene rather than 1-butene.
The presence of hydrocarbon radicals in the molecules of alcohols cannot but affect the chemical properties of alcohols.
The chemical properties of alcohols caused by the hydrocarbon radical are different and depend on its nature. So, all alcohols burn; unsaturated alcohols containing a double C=C bond in the molecule enter into addition reactions, undergo hydrogenation, add hydrogen, react with halogens, for example, decolorize bromine water, etc.
Methods of obtaining
1. Hydrolysis of haloalkanes. You already know that the formation of haloalkanes when alcohols interact with hydrogen halogens is a reversible reaction. Therefore, it is clear that alcohols can be obtained by hydrolysis of haloalkanes - the reaction of these compounds with water.
Polyhydric alcohols can be obtained by hydrolysis of haloalkanes containing more than one halogen atom per molecule.
2. Hydration of alkenes - the addition of water at the tg bond of an alkene molecule - is already familiar to you. Hydration of propene leads, in accordance with Markovnikov’s rule, to the formation of a secondary alcohol - propanol-2
HE
l
CH2=CH-CH3 + H20 -> CH3-CH-CH3
propene propanol-2
3. Hydrogenation of aldehydes and ketones. You already know that the oxidation of alcohols under mild conditions leads to the formation of aldehydes or ketones. It is obvious that alcohols can be obtained by hydrogenation (reduction with hydrogen, addition of hydrogen) of aldehydes and ketones.
4. Oxidation of alkenes. Glycols, as already noted, can be obtained by oxidation of alkenes with an aqueous solution of potassium permanganate. For example, ethylene glycol (ethanediol-1,2) is formed by the oxidation of ethylene (ethene).
5. Specific methods for producing alcohols. Some alcohols are obtained using methods that are unique to them. Thus, methanol is produced industrially by the interaction of hydrogen with carbon monoxide (II) (carbon monoxide) at elevated pressure and high temperature on the surface of a catalyst (zinc oxide).
The mixture of carbon monoxide and hydrogen required for this reaction, also called (think about why!) “synthesis gas,” is obtained by passing water vapor over hot coal.
6. Fermentation of glucose. This method of producing ethyl (wine) alcohol has been known to man since ancient times.
Let's consider the reaction of producing alcohols from haloalkanes - the hydrolysis reaction of halogenated hydrocarbons. It is usually carried out in an alkaline environment. The released hydrobromic acid is neutralized, and the reaction proceeds almost to completion.
This reaction, like many others, proceeds through the mechanism of nucleophilic substitution.
These are reactions the main stage of which is substitution, which occurs under the influence of a nucleophilic particle.
Let us recall that a nucleophilic particle is a molecule or ion that has a lone electron pair and is capable of being attracted to a “positive charge” - regions of the molecule with a reduced electron density.
The most common nucleophilic species are ammonia, water, alcohol, or anions (hydroxyl, halide, alkoxide ion).
The particle (atom or group of atoms) that is replaced by a reaction with a nucleophile is called a leaving group.
The replacement of the hydroxyl group of an alcohol with a halide ion also occurs through the mechanism of nucleophilic substitution:
CH3CH2OH + HBr -> CH3CH2Br + H20
Interestingly, this reaction begins with the addition of a hydrogen cation to the oxygen atom contained in the hydroxyl group:
CH3CH2-OH + H+ -> CH3CH2- OH
Under the influence of an attached positively charged ion, the C-O bond shifts even more towards oxygen, and the effective positive charge on the carbon atom increases.
This leads to the fact that nucleophilic substitution with a halide ion occurs much more easily, and a water molecule is split off under the action of the nucleophile.
CH3CH2-OH+ + Br -> CH3CH2Br + H2O
Preparation of ethers
When sodium alkoxide reacts with bromoethane, the bromine atom is replaced by an alkoxide ion and an ether is formed.
The nucleophilic substitution reaction in general can be written as follows:
R - X +HNu -> R - Nu +HX,
if the nucleophilic particle is a molecule (HBr, H20, CH3CH2OH, NH3, CH3CH2NH2),
R-X + Nu - -> R-Nu + X - ,
if the nucleophile is an anion (OH, Br-, CH3CH2O -), where X is a halogen, Nu is a nucleophilic particle.
Individual representatives of alcohols and their significance
Methanol (methyl alcohol CH3OH) is a colorless liquid with a characteristic odor and a boiling point of 64.7 °C. Burns with a slightly bluish flame. The historical name of methanol - wood alcohol - is explained by one of the methods of its production - distillation of hard wood (Greek - wine, to get drunk; substance, wood).
Methanol is very poisonous! It requires careful handling when working with it. Under the action of the enzyme alcohol dehydrogenase, it is converted in the body into formaldehyde and formic acid, which damage the retina, cause death of the optic nerve and complete loss of vision. Ingestion of more than 50 ml of methanol causes death.
Ethanol (ethyl alcohol C2H5OH) is a colorless liquid with a characteristic odor and a boiling point of 78.3 °C. Flammable Mixes with water in any ratio. The concentration (strength) of alcohol is usually expressed as a percentage by volume. “Pure” (medicinal) alcohol is a product obtained from food raw materials and containing 96% (by volume) ethanol and 4% (by volume) water. To obtain anhydrous ethanol - “absolute alcohol”, this product is treated with substances that chemically bind water (calcium oxide, anhydrous copper(II) sulfate, etc.).
In order to make alcohol used for technical purposes unsuitable for drinking, small amounts of difficult-to-separate toxic, bad-smelling and disgusting-tasting substances are added to it and tinted. Alcohol containing such additives is called denatured or denatured alcohol.

Ethanol is widely used in industry for the production of synthetic rubber, medicines, is used as a solvent, is part of varnishes and paints, and perfumes. In medicine, ethyl alcohol is the most important disinfectant. Used for preparing alcoholic drinks.
When small amounts of ethyl alcohol enter the human body, they reduce pain sensitivity and block inhibition processes in the cerebral cortex, causing a state of intoxication. At this stage of the action of ethanol, water separation in the cells increases and, consequently, urine formation accelerates, resulting in dehydration of the body.
In addition, ethanol causes dilation of blood vessels. Increased blood flow in the skin capillaries leads to redness of the skin and a feeling of warmth.
In large quantities, ethanol inhibits brain activity (inhibition stage) and causes impaired coordination of movements. An intermediate product of ethanol oxidation in the body, acetaldehyde, is extremely toxic and causes severe poisoning.
Systematic consumption of ethyl alcohol and drinks containing it leads to a persistent decrease in brain productivity, death of liver cells and their replacement with connective tissue - liver cirrhosis.
Ethanediol-1,2 (ethylene glycol) is a colorless viscous liquid. Poisonous. Unlimitedly soluble in water. Aqueous solutions do not crystallize at temperatures significantly below 0 °C, which makes it possible to use it as a component of non-freezing coolants - antifreeze for internal combustion engines.
Propanetriol-1,2,3 (glycerol) is a viscous, syrupy liquid with a sweet taste. Unlimitedly soluble in water. Non-volatile. As a component of esters, it is found in fats and oils. Widely used in cosmetics, pharmaceutical and food industries. In cosmetics, glycerin plays the role of an emollient and soothing agent. It is added to toothpaste to prevent it from drying out. Glycerin is added to confectionery products to prevent their crystallization. It is sprayed onto tobacco, in which case it acts as a humectant that prevents the tobacco leaves from drying out and crumbling before processing. It is added to adhesives to prevent them from drying out too quickly, and to plastics, especially cellophane. In the latter case, glycerin acts as a plasticizer, acting like a lubricant between polymer molecules and thus giving plastics the necessary flexibility and elasticity.
1. What substances are called alcohols? By what criteria are alcohols classified? What alcohols should be classified as butanol-2? butene-Z-ol-1? penten-4-diol-1,2?
2. Write down the structural formulas of the alcohols listed in Exercise 1.
3. Are there quaternary alcohols? Explain your answer.
4. How many alcohols have the molecular formula C5H120? Make up the structural formulas of these substances and name them. Can this formula only correspond to alcohols? Make up the structural formulas of two substances that have the formula C5H120 and are not alcohols.
5. Name the substances whose structural formulas are given below: 
6. Write the structural and empirical formulas of a substance whose name is 5-methyl-4-hexen-1-inol-3. Compare the number of hydrogen atoms in the molecule of this alcohol with the number of hydrogen atoms in the molecule of an alkane with the same number of carbon atoms. What explains this difference?
7. Comparing the electronegativity of carbon and hydrogen, explain why the O-H covalent bond is more polar than the C-O bond.
8. Which alcohol do you think - methanol or 2-methylpropanol-2 - will react more actively with sodium? Explain your answer. Write down equations for the corresponding reactions.
9. Write down reaction equations for the interaction of 2-propanol (isopropyl alcohol) with sodium and hydrogen bromide. Name the reaction products and indicate the conditions for their implementation.
10. A mixture of propanol-1 and propanol-2 vapors was passed over heated copper(P) oxide. What reactions could occur in this case? Write down equations for these reactions. What classes of organic compounds do their products belong to?
11. What products can be formed during the hydrolysis of 1,2-dichloropropanol? Write down equations for the corresponding reactions. Name the products of these reactions.
12. Write down equations for the reactions of hydrogenation, hydration, halogenation and hydrohalogenation of 2-propenol-1. Name the products of all reactions.
13. Write down equations for the interaction of glycerol with one, two and three moles of acetic acid. Write the equation for the hydrolysis of an ester - the product of the esterification of one mole of glycerol and three moles of acetic acid.
14*. When the primary saturated monohydric alcohol reacted with sodium, 8.96 liters of gas (n.e.) were released. When the same mass of alcohol is dehydrated, an alkene weighing 56 g is formed. Determine all possible structural formulas of the alcohol.
15*. The volume of carbon dioxide released during the combustion of saturated monohydric alcohol is 8 times greater than the volume of hydrogen released by the action of excess sodium on the same amount of alcohol. Establish the structure of an alcohol if it is known that its oxidation produces a ketone.
Use of alcohols
Since alcohols have various properties, their area of application is quite wide. Let's try to figure out where alcohols are used.

Alcohols in the food industry
Alcohol such as ethanol is the basis of all alcoholic beverages. And it is obtained from raw materials that contain sugar and starch. Such raw materials can be sugar beets, potatoes, grapes, as well as various cereals. Thanks to modern technologies, during the production of alcohol, it is purified from fusel oils.
Natural vinegar also contains ethanol-based raw materials. This product is obtained through oxidation by acetic acid bacteria and aeration.
But in the food industry they use not only ethanol, but also glycerin. This food additive promotes the connection of immiscible liquids. Glycerin, which is part of liqueurs, can give them viscosity and a sweet taste.
Also, glycerin is used in the manufacture of bakery, pasta and confectionery products.
Medicine
In medicine, ethanol is simply irreplaceable. In this industry, it is widely used as an antiseptic, as it has properties that can destroy microbes, delay painful changes in the blood and prevent decomposition in open wounds.
Ethanol is used by medical workers before performing various procedures. This alcohol has disinfecting and drying properties. During artificial ventilation of the lungs, ethanol acts as an antifoam. Ethanol can also be one of the components of anesthesia.
When you have a cold, ethanol can be used as a warming compress, and when cooling, as a rubbing agent, since its substances help restore the body during heat and chills.
In case of poisoning with ethylene glycol or methanol, the use of ethanol helps reduce the concentration of toxic substances and acts as an antidote.
Alcohols also play a huge role in pharmacology, as they are used to prepare healing tinctures and all kinds of extracts.
Alcohols in cosmetics and perfumes

In perfumery, it is also impossible to do without alcohol, since the basis of almost all perfume products is water, alcohol and perfume concentrate. Ethanol in this case acts as a solvent for fragrant substances. But 2-phenylethanol has a floral scent and can replace natural rose oil in perfumery. It is used in the manufacture of lotions, creams, etc.
Glycerin is also the base for many cosmetics, as it has the ability to attract moisture and actively moisturize the skin. And the presence of ethanol in shampoos and conditioners helps moisturize the skin and makes it easier to comb hair after washing your hair.
Fuel

Well, alcohol-containing substances such as methanol, ethanol and butanol-1 are widely used as fuel.
Thanks to the processing of plant materials such as sugar cane and corn, it was possible to obtain bioethanol, which is an environmentally friendly biofuel.
Recently, the production of bioethanol has become popular in the world. With its help, the prospect of renewing fuel resources appeared.
Solvents, surfactants
In addition to the applications of alcohols already listed, it can be noted that they are also good solvents. The most popular in this area are isopropanol, ethanol, and methanol. They are also used in the production of bit chemicals. Without them, proper care of a car, clothing, household utensils, etc. is not possible.
The use of alcohols in various areas of our activities has a positive effect on our economy and brings comfort to our lives.